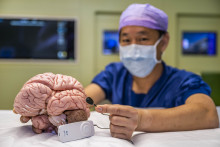
Packed in a sterile, transparent blister wrap, the small electrode seems simple: just a ten centimeters-long wire, attached to a flat metal square of about one centimeter. But its simple looks are deceiving: this device can measure brain activity 24/7, generating massive amounts of relevant EEG data that may hold clues to better treat epilepsy.
‘We place this electrode under the skin of a patient just above the ear, where it measures the EEG of the temporal lobe,’ neurosurgeon Kuan Kho explains, while pointing at a relatively large lobe of a plastic brain model. ‘This brain region is frequently involved in epilepsy attacks.’ The EEG data measured by the electrode are subsequently picked-up by a small receiver that is placed on the skin behind the ear, and transferred to a small, portable box that is only about seven by three centimeters in size.’

While Kho, affiliated to Medisch Spectrum Twente hospital (MST), is the main person responsible for the implantation of the electrode, UT professor Michel van Putten focuses on developing software to analyze the EEG data that are collected. In addition to working as a professor at UT Tech Med Center, where he develops software for data analyses, Van Putten also heads the clinical neurophysiology department at MST in Enschede.
Function loss
Epilepsy is a neurological disorder, where normal brain function is disrupted by uncontrolled, abnormally synchronized activity of specific brain areas. The symptoms depend on the particular brain areas affected, for example, abnormal perception, muscle jerks or loss of consciousness. ‘You can compare the healthy brain with a well-functioning team of collaborating people with distinct tasks,’ Van Putten explains. ‘During an epileptic attack, one or more areas revolt and start to do their own thing independently of other brain parts.’ During such a ‘revolt’ the cells of the affected brain area start firing uncontrollably, without coordinating their activity with the rest of the brain. This results in a temporary loss of function, and is often followed by a seizure. Most seizures are highly unpredictable and, depending on the particular epilepsy syndrome, may occur only once a year, but in extreme cases up to twenty times per day. About one percent of the population suffers from epilepsy.
Some kind of pacemaker
Many epilepsy patients are successfully treated with medication, however, about a third does not respond. In some of these patients, it’s possible to perform a surgery during which the affected brain part is removed. Another treatment option is electrical stimulation of the vagus nerve, a brain nerve that connects different organs to the brain.

‘By surgically applying a ring electrode around the vagus nerve, and stimulating the nerve fibers that enter the brain using some kind of pacemaker, we can successfully treat about half of the patients that don’t respond to medication,’ Van Putten says. ‘Although we don’t exactly understand how vagus nerve stimulation (VNS) works, it reduces the seizure frequency with at least fifty percent in about 50-70% of the patients.’
Unfortunately, about a quarter of the patients doesn’t respond to VNS. This typically becomes apparent after about a year. This is not only disappointing for the patient, but also associated with significant health care costs, with a price tag between 30.000 and 40.000 euro.
Brain biomarkers
To predict if patients will benefit from VNS, Van Putten and his team are looking for clues in the brain that may forecast if this treatment works before the implantation of the device. Van Putten: ‘We aim to identify brain biomarkers that tell us beforehand if a patient will respond to this treatment and how effective it will be.’ The scientists believe that the activity of the brain, measured as an EEG, may hold such clues. Therefore, the team uses a small electrode, placed just under the skin behind the ear. The electrode, developed in Denmark, can continuously measure brain activity, for more than one year. Van Putten and his team started the clinical trial in December 2020, and at the moment, six patients have been included. To reliably test their hypothesis, they will include forty patients in total.
Massive amount of data
During the second stage, the team will also implant the VNS in the same patients and continue their measurements for up to one year. During this period, it becomes clear if a particular patient responds to the treatment or not. ‘We will compare the EEG before and after VNS, and look for differences in these patterns between patients that didn’t respond to VNS and those that did,’ Van Putten explains. ‘However, to analyze this massive amount of data is a huge scientific challenge. At the UT, we are currently developing self-learning software, artificial intelligence (AI), to do this immense job.’

The development of AI for data analyses is just one aspect of this complicated study, where a variety of disciplines comes together and neurologists, neurosurgeons, technical physicians and engineers work closely together. In addition to Van Putten and neurosurgeon Kuan Kho, team members include technical physician Marleen Tjepkema, neurologists Anne Marthe Meppelink and Jacqueline Ardesch, and several master and PhD students. ‘The project will be successful if the team can identify EEG biomarkers that will predict the effect of VNS in patients with epilepsy,’ Van Putten says. ‘If we succeed, we will first implant the small electrode in patients who are potential candidates for VNS. Using the recorded brain signals, we can then make a personalized advice regarding VNS treatment.’